Software Supply Chain Actionable SBOMs Cyber Compliance
SBOM Lifecycle Management for mission-critical software and devices
Vigilant Ops is the only platform that manages the entire Software Bill of Materials (SBOM) lifecycle, from generation to monitoring to management.
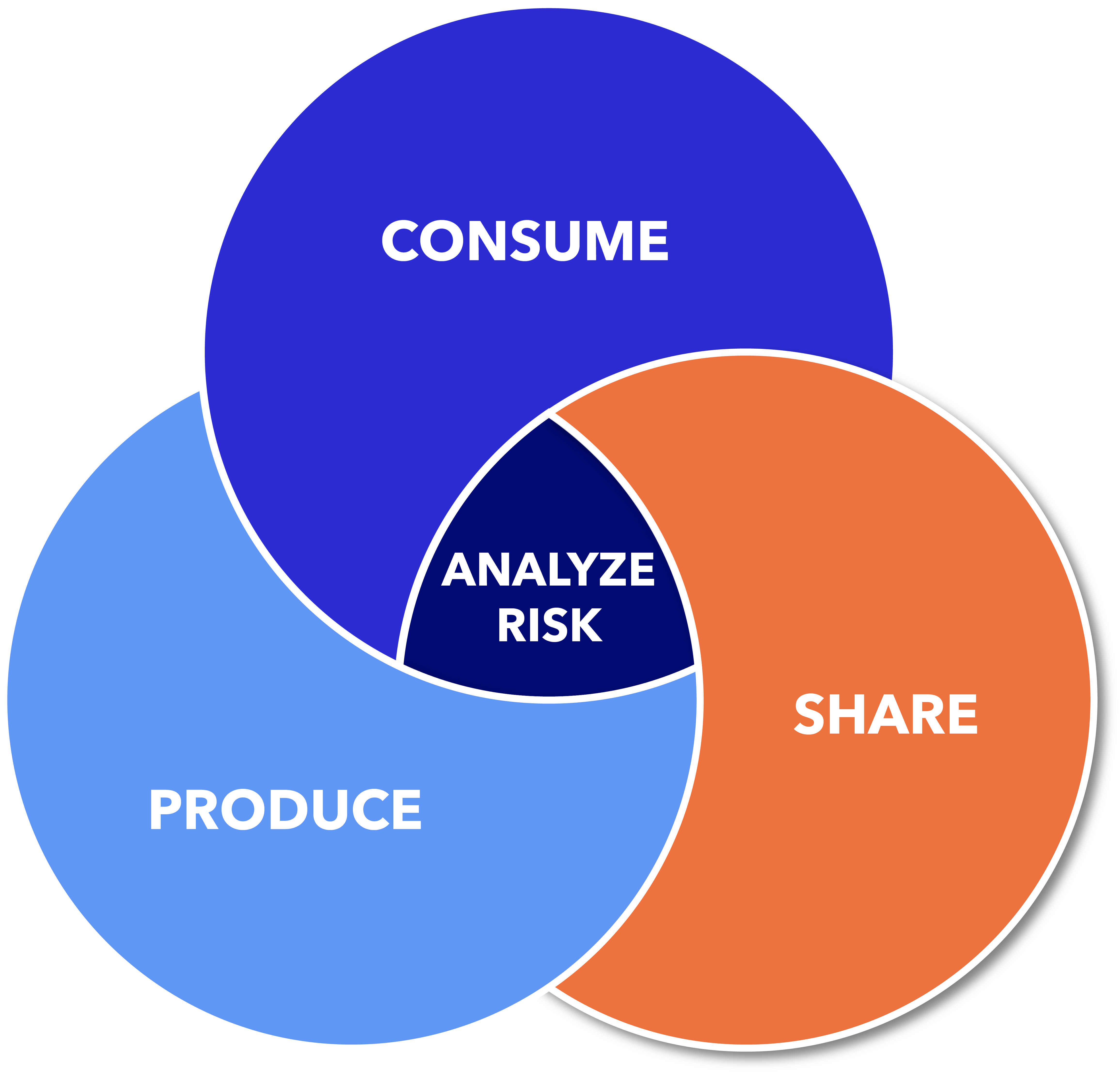
No matter where you are on your SBOM journey, we can help.
SBOMs are more than just a checklist—they’re a tool securing your entire software supply chain.
We offer modular, affordable platform options that scale with you. Whether you’re building your first SBOM or managing thousands, our solutions are tailored to fit your organizational size and compliance goals. Manage SBOMs effectively and strengthen your product security every step of the way.
Are you ready to take control of your SBOM journey?
Know when a vulnerability affects your business.
See what’s in your software, and then do something about it. Vigilant Ops keeps your SBOMs in one place, automatically searches for new vulnerabilities, and helps you take action to meet compliance standards that keep everyone safe.
SBOM Generation
Instantly build and certify new SBOMs or ingest third-party SBOMs
SBOM Monitoring
Easily address vulnerabilities with continuous monitoring and timely alerts
SBOM Management
Organize and distribute SBOMs from a single central dashboard
SBOM Compliance
Meet the latest cybersecurity standards and regulatory requirements with our one-click compliance
Secure your
software
supply chain
Can you trust the technology you buy, sell, and build? Secure software depends on a secure supply chain. Vigilant Ops generates and manages SBOMs to track every component and vulnerability.
Stay compliant
without trying
Vigilant Ops addresses complex challenges that medical device manufacturers and customers face, including the FDA’s latest industry guidance. Use Vigilant Ops to meet or exceed all FDA requirements and security standards.
- Meet premarket and postmarket guidance
- Submit FDA documentation
- Prevent RTAs and product recalls
What customers are saying...
"My team relies on Vigilant Ops to generate and maintain our medical device SBOMs. It eliminates tons of manual effort!"
Head of Medical Device Cybersecurity, Bayer“We’ve been very happy with our service from Vigilant Ops—and so was the FDA during our recent 510(k) submission. Their platform gave us exactly what we needed to streamline SBOM generation and produce documentation while meeting regulatory expectations with confidence."
Senior Executive for a Startup Medical Device Manufacturer"Now, our engineers spend their time on development instead of monitoring security sources."
Application Security Manager, Ascensia"I was spending 15% of my engineering resource time on SBOMs. With [Vigilant Ops], I have reduced that to nearly zero!"
Chief Information Security Officer, Global Medical Device Manufacturer"There isn't a better solution. It makes SBOM creation simple and has all the functionality to keep your products safe."
Director at a Global Medical Instrumentation Company